Highlight: efficient noise reduction cleaning room cabin, cleaning room cabin with led lighting
|
액세서리:
|
팬, 필터, 조명, 소켓
|
공기 속도:
|
0.45m/s
|
|
온도:
|
18-25℃
|
조명:
|
LED
|
|
공기 흐름:
|
층류 흐름
|
Air Purification:
|
HEPA Filter
|
|
구조:
|
모듈식
|
습도:
|
30%-60%
|
제품 설명:
GMP Device Cleanroom Solutions
(1) It Is Crucial To Maintain a Contaminant-Free Clean Environment When Manufacturing Devices Such As Biological Implants.This Starts With The Clean Room Design And Management Systems. The Clean Room Design Prevents Outside Contamination, While Regular Maintenance And Sanitation Upkeep Minimize The Risk Of Contamination Originating Within The Clean Room Itself.
(2) Implants That Are Non-Sterile Or Are Awaiting Sterilization Should Be Subject To Purification And Clean Packaging Processes. This Will Allow For Uniformity And Control Over Product Quality. When Constructing a GMP Instrument Clean Room, a Location Should Be Designated For The Packaging Process, And Processes Should Be Implemented To Ensure That Products Avoid Contamination Risk At All Times. For Further Reference, Please See The YY0033-2000 Standards.
(3) Procedures Must Be Developed To Control Environments In Which There Exists a Contamination Risk To Products. This Includes Any Time Personnel Makes Contact With a Product, Including When Products Are Moved From One Location To Another.

기능:
- 제품 이름: 클린룸 부스
- Structure: Modular
- Size: Customized
- Pressure: Positive/Negative
- 애플리케이션 의료, 전자, 식품
- Temperature: 18-25℃
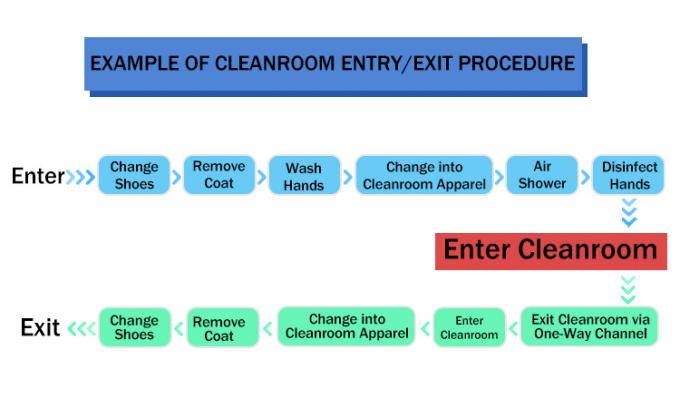
International GMP Instrument Cleanroom Standards
International Standard: ISO/DIS 14644
Chinese Standards: GB50073, GB50591, GB 50243
American Standards: GMP-97, GMP-98, FS209E

Considerations for GMP Device Cleanrooms
(1) Carefully consider all construction materials to be used.
(2) Thoroughly examine your plans for design, installation, testing and maintenance
(3) Do not neglect to consider details–such as placement, size, and model–that concern the air purification systemTemperature and Relative Humidity
Except for certain special circumstances, the temperature of GMP instrument cleanrooms should be calibrated between 18~28℃,with a relatively humidity of 45-65%. If you find that you are out of range for these standards, consider which instruments in the clean room may be acting as a heat source.
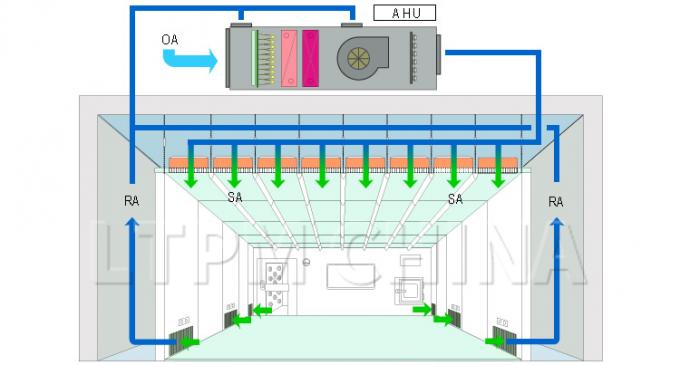
Air Velocity, Air Change Rate (ACR), and Static Pressure
The air change rate of a clean room is a function of the volume of air circulated into the cleanroom and the area of the clean room.Differences in static pressure result from differences in the the amount of air circulating through the air return vs. exhaust system vents. All of these variables can be adjusted through manipulation of fan speed and/or opening/closing the main vents. These can be adjusted for the entire system or for certain areas depending on the situation.Keeping Out Dust, Bacteria, and Other Contaminants
Indications that your clean room may not be up to standard include falling out of standard in basic areas such as temperature,relative humidity and ACR. If these variables are out of standard, it is likely that your cleanroom is contaminated to a degree that is out of standard.Proper calibration of the above conditions forms a sort of “microclimate” that is inhospitable to contaminants such as dust particles and bacteria. Meeting standards is a result of proper maintenance of the cleanroom. Regular, thorough testing is the only best practice, especially in a field where product contamination can lead to serious consequences. Besides designing your clean room, E-Clean can advise on how to implement management systems to maintain your clean room and minimize the risk for contamination.
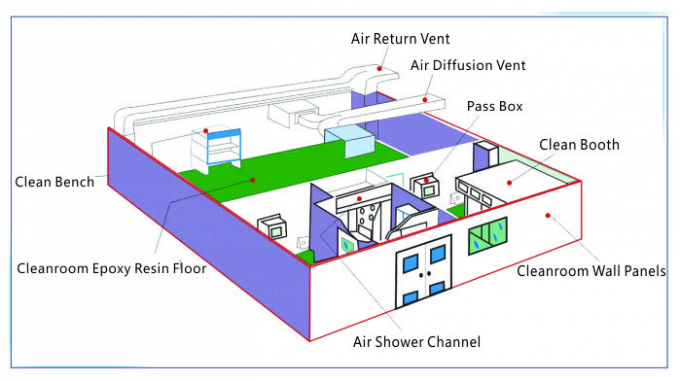
|
|
|
|
|
|
|
|
Aluminum frame/Baking steel
plaxe/lnsulaxing glass window
|
Aluminum frame/Baking sieel
plate/lnsulating glass window
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
If there are other size requirements, it can be customized
Biological Science Modular Clean-Room Mainly Engaged In Offering Full Service For System Clean Room Engineering, Including Product Manufacturing, Equipment Transport, Construction Control, Engineering Acceptance Test, Etc. The Engineering Contains 4 Sectors, Decoration, HVAC, Plumbing And Electrical Appliances, Which Includes The Installation Of Process Pipeline, Water Supply And Drainage Pipeline And Purifying Ventilation Duct, Power Distribution Of Purification Product And Process Equipment, Security Control, Whole Plant Centralized Control, Etc, And Which Are Widely Used In Food, Electronics, And So On. Biological Science Modular Clean-Room Has a First-Class Engineering Design Team To Work From Initial Engineering Design, Further Optimize The Drawings According To Practical Construction Experience, And Even Customize For Clients. In The Whole Construction Process, Biological Science Modular Clean-Room Strictly Follows 4Q Standard To Monitor All Construction Nodes, Applies 3 Level Control From Plant Construction To Completion And Uses Advanced Equipment To Precisely Test The Later Project. The Company Processes Advanced ERP System And Ensures The Engineering, From Quality To Management, As High As International Level.

지원 및 서비스:
Technical support and services for Clean Room Booth include:
- 24/7 customer service
- On-site installation and setup
- Product operation and maintenance training
- Software and firmware updates
- Troubleshooting and diagnostic support
- Parts and components replacement and repair
- Extended warranties and maintenance contracts
Packing and Shipping:
Packaging and Shipping for Clean Room Booth:
- The clean room booth will be shipped in a single box.
- The box should be secured properly and handled with care.
- The box will be labeled with the product name and shipped from the factory.
- The box should be placed on a flat surface to avoid any damage to the booth.
- The box should be opened and the booth should be checked for any damage before installation.






