Search the whole station

|
Accessories:
|
Fan, Filter, Light, Socket
|
Air Velocity:
|
0.45m/s
|
|
Temperature:
|
18-25℃
|
Lighting:
|
LED
|
|
Air Flow:
|
Laminar Flow
|
Air Purification:
|
HEPA Filter
|
|
Structure:
|
Modular
|
Humidity:
|
30%-60%
|
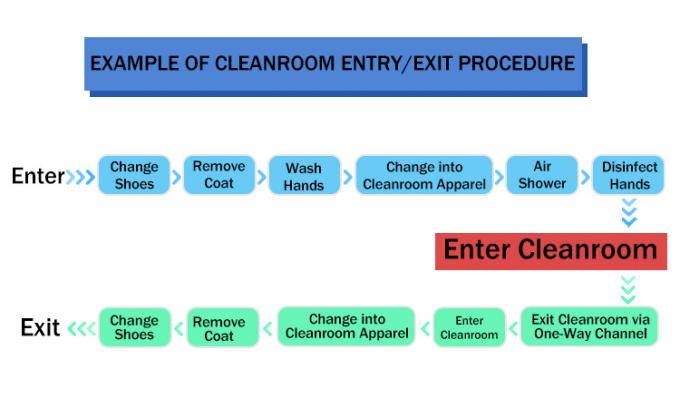
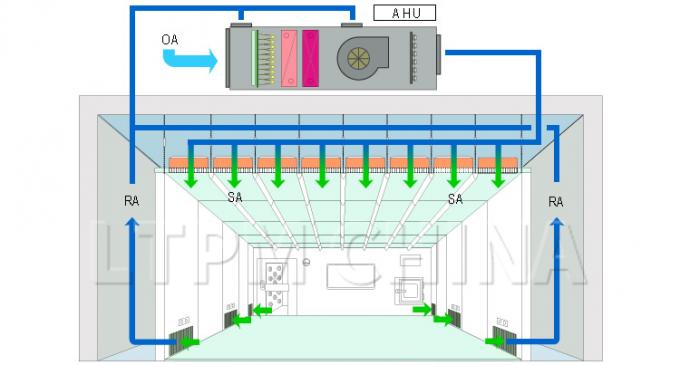
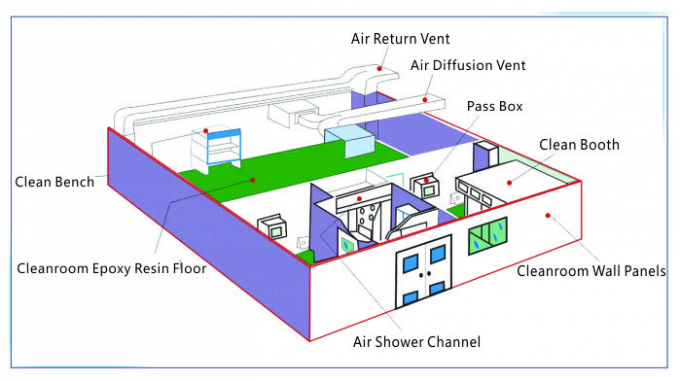
(1) It Is Crucial To Maintain a Contaminant-Free Clean Environment When Manufacturing Devices Such As Biological Implants.This Starts With The Clean Room Design And Management Systems. The Clean Room Design Prevents Outside Contamination, While Regular Maintenance And Sanitation Upkeep Minimize The Risk Of Contamination Originating Within The Clean Room Itself.
(2) Implants That Are Non-Sterile Or Are Awaiting Sterilization Should Be Subject To Purification And Clean Packaging Processes. This Will Allow For Uniformity And Control Over Product Quality. When Constructing a GMP Instrument Clean Room, a Location Should Be Designated For The Packaging Process, And Processes Should Be Implemented To Ensure That Products Avoid Contamination Risk At All Times. For Further Reference, Please See The YY0033-2000 Standards.
(3) Procedures Must Be Developed To Control Environments In Which There Exists a Contamination Risk To Products. This Includes Any Time Personnel Makes Contact With a Product, Including When Products Are Moved From One Location To Another.


|
Area
|
15㎡
|
30㎡
|
50㎡
|
100㎡
|
|
Material
|
Aluminum frame/Baking steel
plaxe/lnsulaxing glass window
|
Aluminum frame/Baking sieel
plate/lnsulating glass window
|
||
|
FFU class 1-100
|
12pcs
|
24pcs
|
48pcs
|
96pcs
|
|
FFU class-1K
|
3pcs
|
6pcs
|
9pcs
|
18pcs
|
|
FFU class-10K
|
2pcs
|
4pcs
|
6pcs
|
12pcs
|
|
Speed (m/s)
|
0.45m/s ±20%
|
0.45m/s ±20%
|
||
|
Temperature(optional)
|
18-28°C
|
|||
|
Humidity(opiional)
|
50-70%
|
50-70%
|
||
|
Illumination
|
400-800LUX
|
|||
|
Power
|
2.4-7kw
|
4.8-10kw
|
9.6-19kw
|
19.2-30kw
|

Technical support and services for Clean Room Booth include:
Packaging and Shipping for Clean Room Booth:
Highlight: clean room booth oem, clean room booth class 100, clean booth class 100 Temperatur……

Highlight: industrial grade clean room booth, contamination control clean room booth, air distribu……
Highlight: ISO6 Soft Curtain, Anti-static Soft Curtain, Clean Room Soft Curtain Product Name: ……

The (SS-MAC) is an affordable and adaptive Clean room unit. It is packaged as a DIY (do-it-yoursel……

Laminar Flow Clean Booth provides a sterile working environment. The Laminar Flow Booth is continu……

Clean booths are used for either localized or advanced particle cleaning to achieve cleanroom stan……

Product Introduction A clean room is a simple purification device with low investment, high purifi……
A hardwall cleanroom for medical device manufacturing requires precision at every intersection, ……
This website uses cookies to improve your browsing experience. By continuing to use this site, you accept the use of our cookies. Data collected from this website is processed and stored in the United States.